Pharmaceutical-Grade Purity
Complies with international quality standards and meets stringent impurity limits.
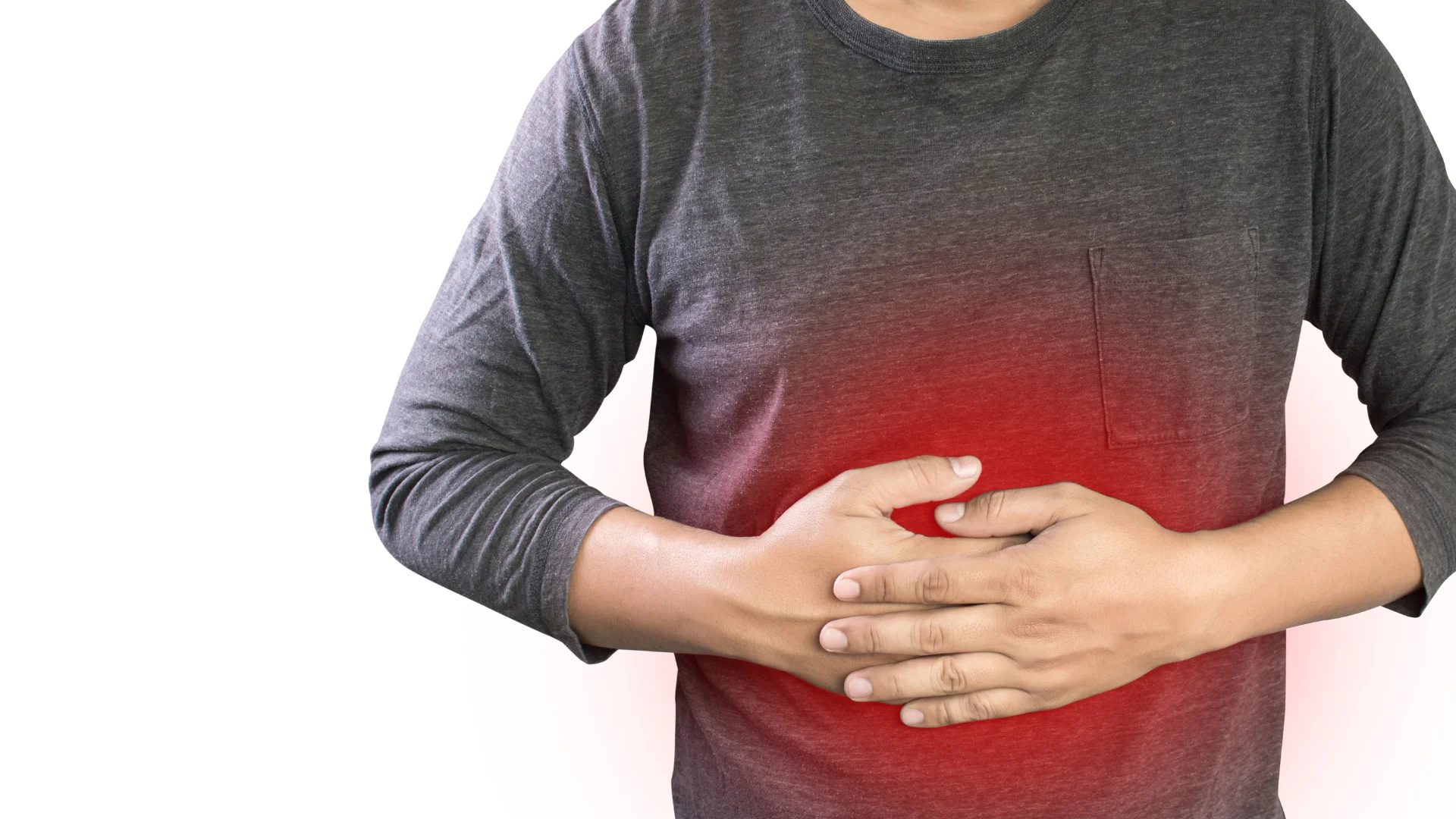
Bismuth Citrate is an organometallic compound used primarily in the pharmaceutical industry due to its excellent therapeutic benefits, particularly in gastrointestinal treatments. Known for its anti-ulcer, antimicrobial, and anti-inflammatory properties, Bismuth Citrate plays a key role in the treatment of Helicobacter pylori infections, peptic ulcers, and other gastric disorders. At Heni Chemicals, we manufacture high-purity Bismuth Citrate under strict GMP conditions, ensuring optimal safety, consistency, and performance across every batch. Our product is widely used in both domestic and international pharmaceutical formulations, R&D projects, and industrial applications that demand reliable, traceable quality.
Complies with international quality standards and meets stringent impurity limits.
Produced in audited, ISO-certified facilities with traceable documentation and batch integrity.

Used in combination therapies for H. pylori eradication and ulcer treatment.
Designed for ease of formulation, uniform blending, and better bioavailability.
Offers good shelf life and chemical stability under standard storage conditions.

Available in multiple grades and pack sizes tailored to end-use requirements.
CoA, MSDS, and full documentation provided with every shipment for regulatory compliance.
Primary active in anti-ulcer and gastroprotective medications. Used in triple therapy for H. pylori eradication alongside antibiotics and PPIs. Acts as a mucosal protectant, preventing damage from gastric acids.
Investigated for its antimicrobial mechanisms. Studied in newer combination therapies and controlled-release formulations.
Limited usage due to regulatory restrictions, but under evaluation for gut health products in trace levels.
Used as a chemical intermediate in the synthesis of bismuth-based pharmaceuticals or specialty compounds.

By Star-K

Food Safety and Standards Authority of India

Quality Management Systems

Good Manufacturing Practices
| Property | Specification |
|---|---|
| Chemical Name | Bismuth(III) Citrate |
| Molecular Formula | C₆H₅BiO₇ |
| CAS Number | 813-93-4 |
| Molecular Weight | 396.09 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Solubility | Slightly soluble in water; more soluble in acidic solutions |
| Bismuth Content (as Bi) | 35.0% – 37.5% |
Hazards Identification
Handling Guidelines
Storage Recommendations
Spill & Disposal
First Aid Measures
At Heni Chemicals, we understand that quality, reliability, and compliance are non-negotiables for pharmaceutical manufacturers. Here’s why we are your trusted source for Bismuth Citrate:
Full regulatory compliance to meet domestic and global standards.
Proven track record across pharmaceutical and specialty chemical sectors.
Serving startups to multinationals — with consistency in every batch.
We can offer micronized, granular, or coarse grades as per formulation needs.
CoA, MSDS, Stability Data, and Technical Dossiers available upon request.
Seamless delivery across India, the US, Europe, Middle East, and beyond.
Yes, our product is manufactured under GMP guidelines and is suitable for regulated pharmaceutical use.
Absolutely. We offer both standard and customized grades depending on your formulation requirements.
Yes, we export to multiple countries and provide complete regulatory and export documentation.
Standard packaging includes 25 kg fiber drums with liners. Custom pack sizes are available upon request.
We provide a full Certificate of Analysis (CoA), Material Safety Data Sheet (MSDS), TDS (Technical Data Sheet), and stability data where applicable. Upon request, we also support with DMF (Drug Master File) filing or Regulatory Dossiers for regulated markets